Scientific innovations in the areas of vaccines, oncology and rare diseases are front and center of research and development (R&D), and these complex therapies require focused scientific discussions. Data sets are multifaceted and beyond the scope of traditional sales rep engagement and as a result, we are witnessing a growth in medical roles. Additionally, many therapies require getting closer to the patient, especially for rare conditions and the meticulous coordination of cell and gene therapies in the oncology space.
Given the very scientific foundation of these therapies, we see the medical affairs function suddenly elevated from its supporting role to a leading strategic partner as it takes on the ownership of engagement across stakeholders.
Understanding the role of medical affairs in a patient-focused strategy
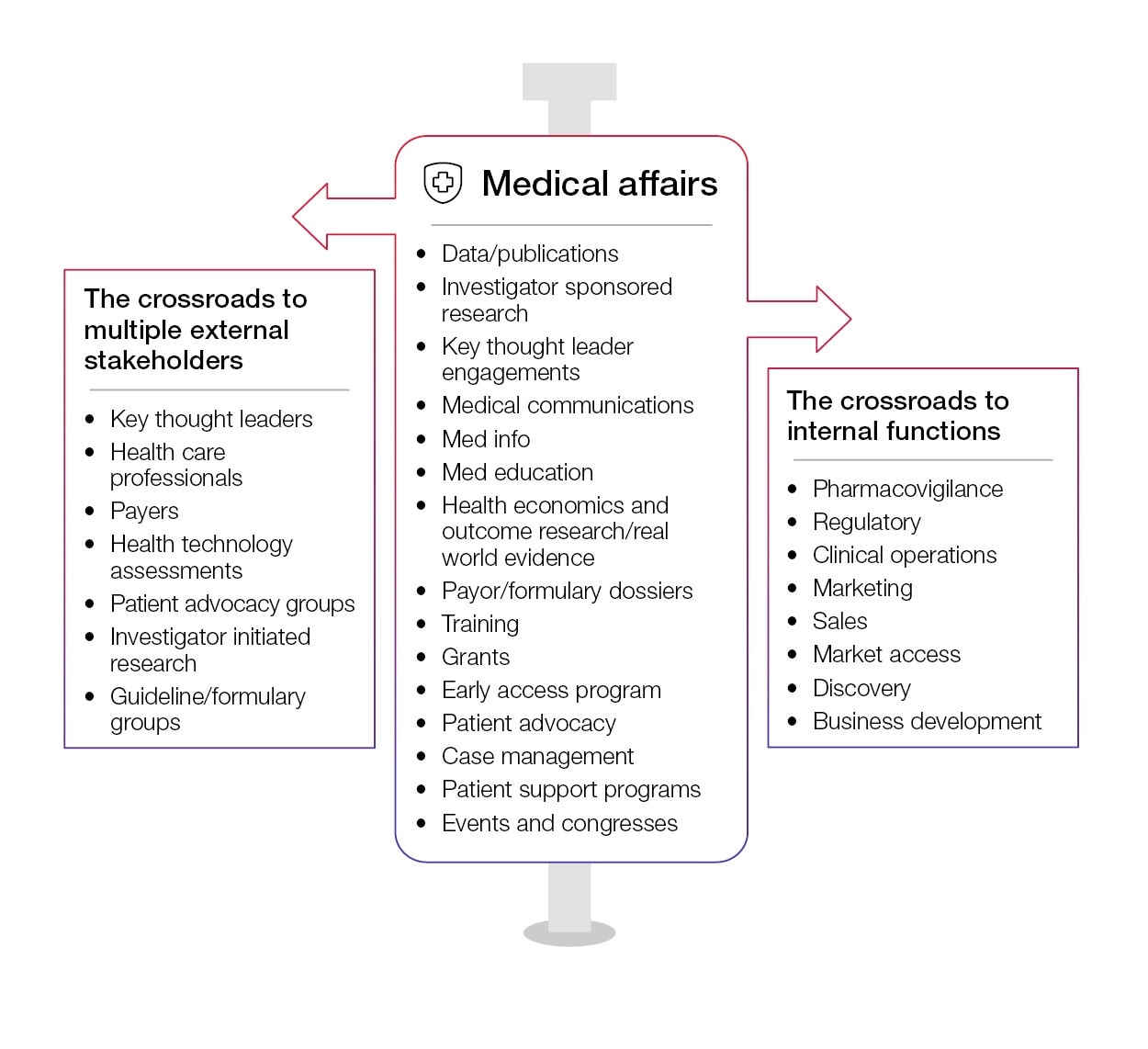
Medical affairs sits in the middle of the R&D and commercial domains. From product development to real-world evidence (RWE), medical affairs is the trusted advisor to business. As such, it should no longer be treated as a supporting function. Today, medical affairs is a key strategic pillar—leading the engagement with providers and payers, while guiding internal business planning opportunities. It is imperative that this function be regarded as central if companies are to live the values of the “patient-focused” strategy. Organizations are beginning to realize that the medical affairs function is taking on a new role that encompasses business skills and a familiarity with many tools and technologies that effectively deliver value to internal and external stakeholders.
Understanding the complex internal and external interactions facilitated by the medical affairs team helps us appreciate the tremendous number of stakeholders and data sources that must be managed.
In all of these interactions, there are some essential and non-negotiable elements: information, processes, compliance, data insights, people and technology. The question is, how are these variables working for medical affairs – a function that is taking a leading role in gathering, analyzing and disseminating scientific data across dozens of stakeholders for different purposes at different times across the life cycle of a product? In this context, significant aspects of medical affairs activities now require updating. For example, how will medical performance management be designed to measure the impact of medical activities in the shifting engagement model? What methodology will be used to define the value creation activities in support of complex innovative therapies?
Traditionally, this supporting function relied heavily on its medical credentials to support all activities. In the new medical affairs world, the success of this function will require its leaders to have strong business knowledge, adaptive leadership styles, and strong technical skills, including digital technology and analytics. Medical leaders will influence business priorities and work with an agile mindset to sense and respond to shifting priorities and risks with speed and governance. This new setting will require updating medical profiles, technology tools upskilling, and implementing the right tools and technology to embed a culture of data and analytics, as well as digital engagement techniques.
The rising role of Medical Science Liaisons (MSLs)
We have begun to see an increase in the roles of MSLs who are now engaging a broader set of healthcare stakeholders. In addition to key opinion leaders (KOL), MSLs are now engaging selected healthcare professionals (HCPs), payers, principal investigators and patient advocacy groups. These discussions are carefully planned, each tailored for specific needs and to the preferences of the individual stakeholder. Quality and sophisticated data presentations are required. Mechanism of Action models and visualizations of data are value adds to payers and providers. Customer relationship management (CRM), data dashboard, patient journeys, processing data gathered from various types of interactions, and so much more, will reshape how medical teams work and the skill sets required.
Based on lessons learned during the pandemic, digital tools will continue to replace many face-to-face interactions, leading to more robust data gathering and insights into each stakeholder’s engagement. Partnering with patients from strategy to solutions across the value chain will be supported by digital tools and applications that enable patients to play a more active role in their disease and treatment.
Similarly, in striving for patient inclusivity, MSLs will work more closely with patient advocacy groups to support the co-creating of treatments with patients; from strategy and policy to clinical trials and evidence-based outcomes. This paradigm shift entails reimaging patient data collection (patient portal—building and hosting registries); data connection (a platform that connects with other data and platforms) and data federation analysis (enabling patient-level data analysis through federated technology for researchers and drug developers).
Medical information: automated, digitized and centralized
Historically, medical information sits everywhere and has many owners. Centralized access and global models of medical affairs have not been given much focus in digital transformation strategies, especially when not addressed from an enterprise level. Given the vision for a leading and agile medical function in the era of specialty medicines, many organizations are looking to automation and digitization of medical information, as well as centralization of data for easy, real-time access. A secure IT environment is essential. Privacy and cybersecurity are expected by the patient and demanded by law and regulation.
Prioritizing investments to optimize experiences and outcomes
In summary, to optimize the experience and outcomes of physicians and patients, medical affairs will be required to elevate its performance across all its activities. It is imperative that investments be prioritized for the right digital initiatives and tools to facilitate this evolution. Equally as important is the recruiting of additional talent, such as data scientists, to support the ongoing complexity of data interpretation and customer inquiries.
Over the next three years, we anticipate that traditional medical activities will be reshaped by cutting-edge digital and analytical tools. The complex scientific innovations are disrupting the traditional approaches to care and reimbursement, which no longer are effective. CRM systems for customer engagement will be enhanced with AI applications for optimal value creation – getting the right message to the right customer at the right time.
Key considerations for elevating the medical affairs function
As biopharma moves to elevate the medical affairs function’s leadership role across stakeholders, both internally and externally, the following factors must be considered to enable a value-driven organization:
- Technology: prioritize use cases to define the right digital tools and platforms, including AI, advanced analytics and cybersecurity.
- Performance management: identify the value-driven medical activities and measure the impact on outcomes.
- Patient focus: address direct patient needs with digital assets that support patients throughout their journey and potentially post-treatment.
- Change management: implement a change management strategy that helps individuals understand the “why” behind engaging in and adopting new processes and tools.
If all of the above are built into an overall strategy, then biopharma will achieve the reimagined go-to-market model where there is precision engagement, favored remote interactions, coordinated HCP interfaces, content that HCPs care about and secure technology-enabled go-to-market decisions.